Pipeline
Our clinical program
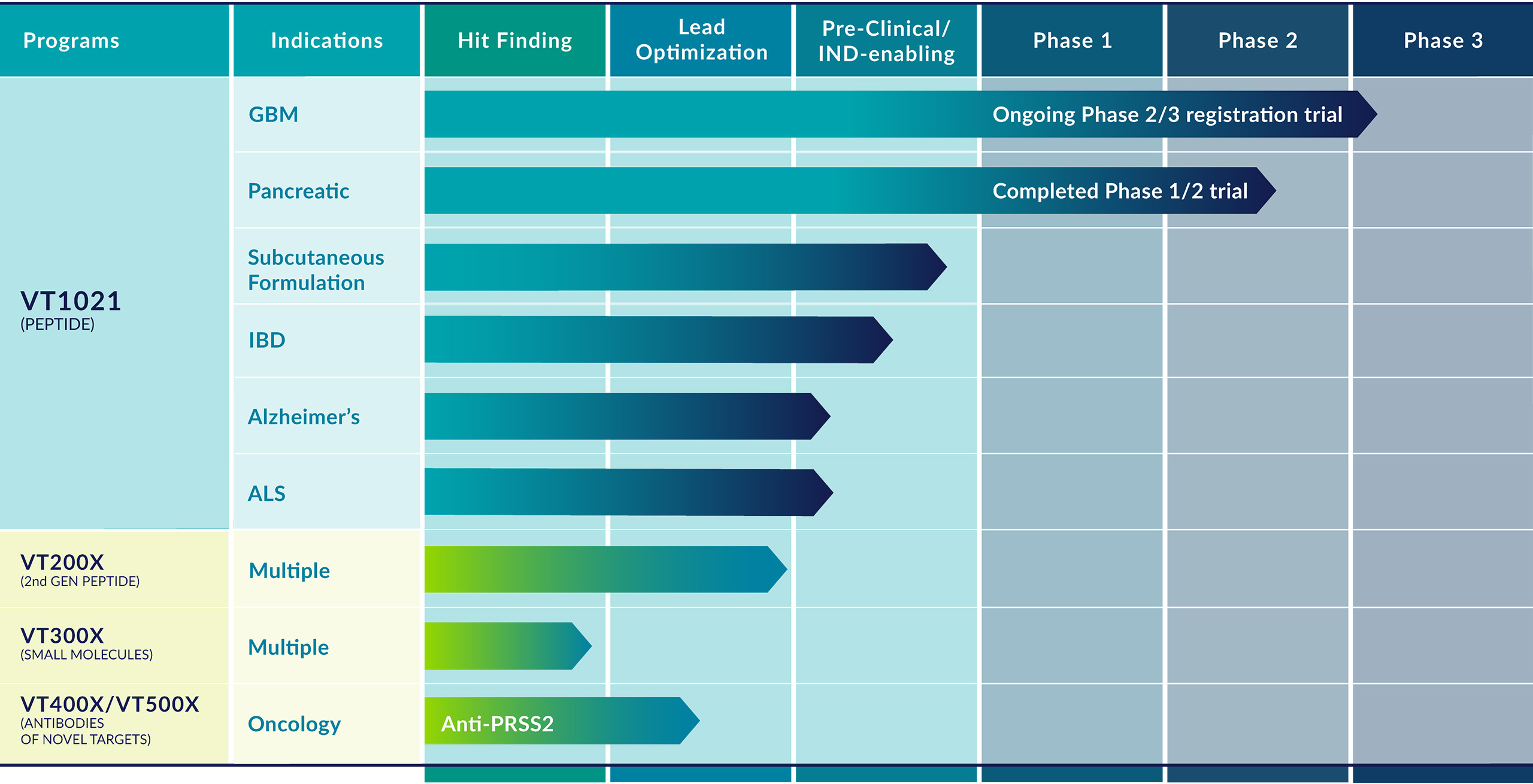
Vigeo Therapeutics is an immunotherapy company focused on developing therapies to fight cancer and inflammation. Vigeo is a leader in understanding the important role endogenous Thrombospondin-1 (TSP-1) plays in maintaining a healthy immune system. In many diseases (Oncology, Alzheimer’s disease, ALS and IBD), the immune system falters as TSP-1 levels drop, allowing disease to progress. Vigeo’s lead program, VT1021, is a small peptide derived from PSAP which induces TSP-1 production, restoring a healthier, more robust disease fighting immune system.
Vigeo is currently enrolling patients as an arm of AGILE, a Phase 3 registration ready clinical trial in Glioblastoma NCT03970447. Previously Vigeo completed a Ph1/2 study of VT101 in solid tumor indications (NCT03364400). In one of the expansion cohorts of the study, VT1021 was tested in recurrent Glioblastoma (rGBM) patients. Of the 22 evaluable rGBM patients dosed, 3 of them achieved Complete Responses (CR), 1 achieved Partial Response (PR, >60% reduction), and 45% of patients showed tumor shrinkage. One of the CR patients continues to be dosed with VT1021 and has been tumor free for over 3 years.
Vigeo has also developed a robust pre-clinical program with VT1021 in inflammatory and neurodegenerative diseases focusing on Alzheimer’s disease, ALS, and IBD.

